How to calculate the number of molecules of an element in a compound if I only know the total mass - Quora
a) Calculate the mass of 0.5 mole of oxygen atoms. (b) Calculate the number of molecules of glucose present in its 90 grams (molecular mass of glucose is 180 u) (c) Calculate
Calculate the number of particles in each of the following: (a) 48 g of Mg (b) 8 g of O2 (c) 0.1 mole of carbon (Atomic mass Mg = 24 u, O =

Calculate the number of molecules in 36gm of H2o - Chemistry - Atoms and Molecules - 14431885 | Meritnation.com
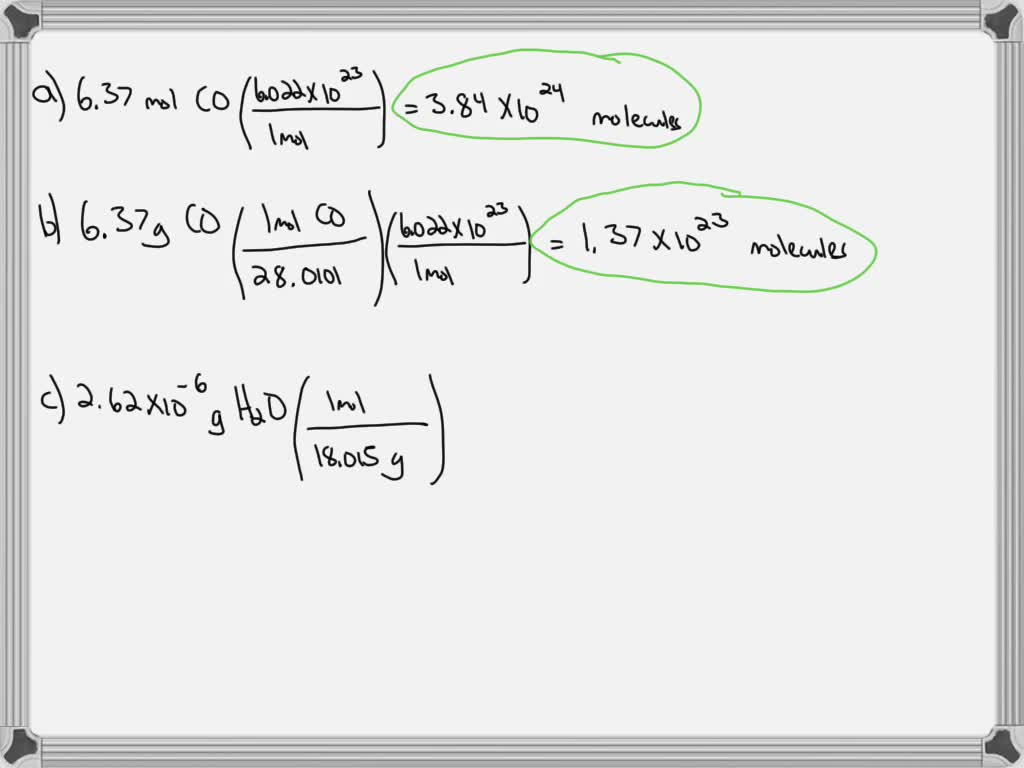
SOLVED: Calculate the number of molecules present in each of the following samples. a. 6.37 mol of carbon monoxide b. 6.37 g of carbon monoxide c. 2.62 × 10^-6 of water d.
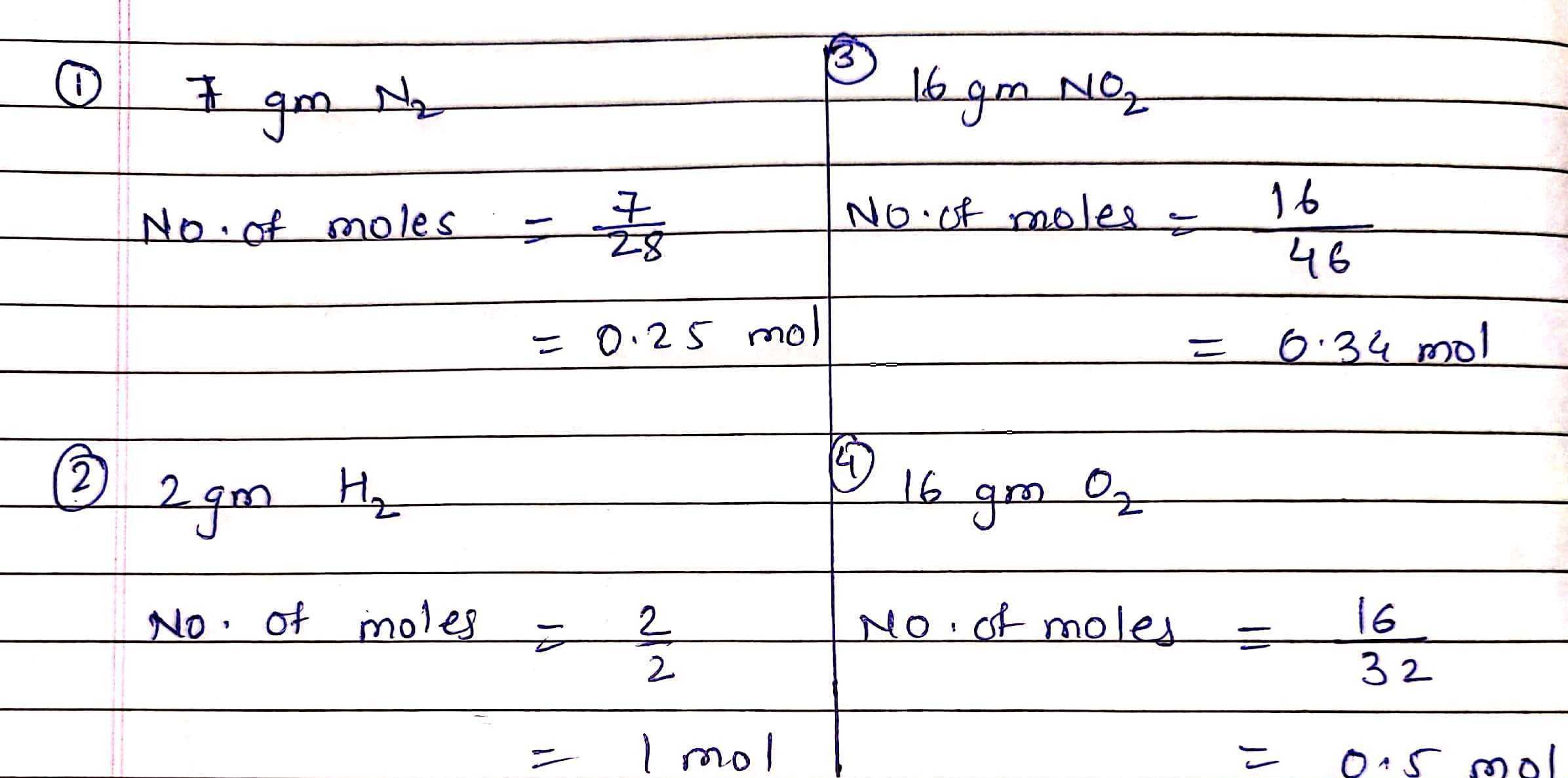
which has maximum number of molecules 1 7 gmn2 2 2 gmh2 3 16 gmno2 4 16 gmo2 8rxwz5pp -Chemistry - TopperLearning.com

How to calculate Z (the number of molecules in a unit cell) for calculating theoretical density? | ResearchGate

SOLVED:Calculate the number of molecules present in each of the following samples. a. 6.37 mol of carbon monoxide b. 6.37 g of carbon monoxide c. 2.62 ×10^-6 of water d. 2.62 ×10^-6

Calculate the number of moles and the number of molecules present in 1.4 g of ethylene gas. What is the volume occupied by the same amount of ethylene?A. 1.25 litresB. 1.12 litresC.

Calculate the number of molecules of sulphur (S8) present in 16 g of solid sulphur (Aton mass of S = 32 )